When injecting gases underground, one subject of critical importance is the safety of freshwater aquifers that provide sources of clean drinking water. The U.S. Safe Drinking Water Act provides numerous rules for subsurface injection to protect these aquifers, but many people still have questions about the safety of injecting CO2 underground. Below are answers to some common concerns the public may have about CO2 injection.
How far underground is CO2 stored?
Captured CO2 is usually stored in reservoirs about a mile underground, although in Texas and the Gulf of Mexico well-suited reservoirs are often as deep as two miles.1How far underground will CO2 be stored? Gulf Coast Carbon Center. https://www.co2facts.org/faqs (accessed 10/24/2020)

Typical depths for CO2 injection are one mile or greater, equivalent to 17 times the height of the Statue of Liberty.
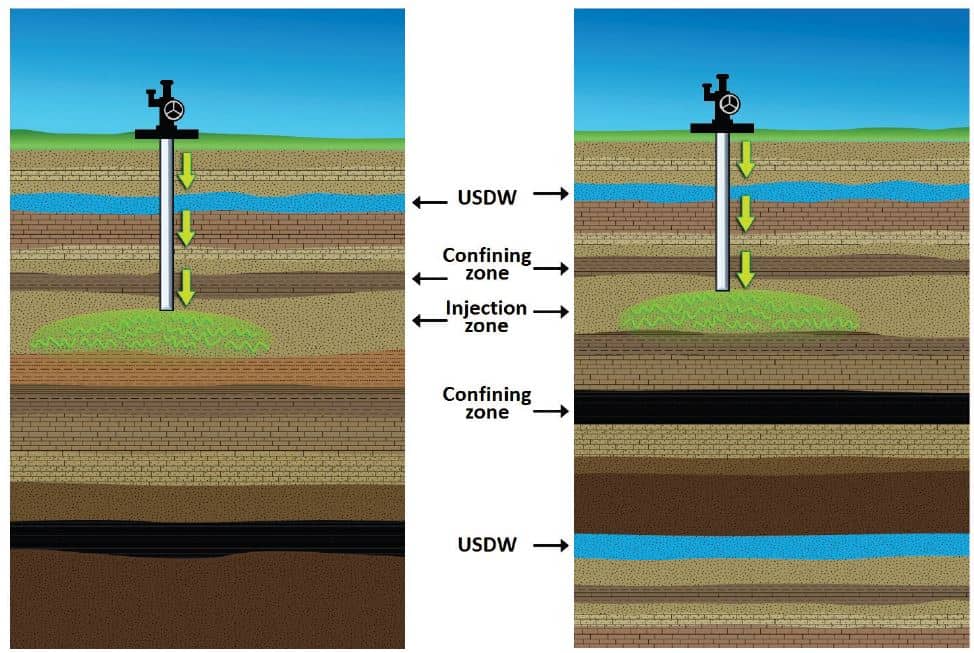
There are two main reasons why we inject CO2 at these depths. The first reason is—it’s the law! The U.S. Safe Drinking Water Act requires injections to occur at significant depths below and geologically isolated from the nearest USDW to avoid the possibility of contamination. To use an example in Texas, the Edwards Aquifer is the source of drinking water for two million people. The aquifer ranges from 300-700 feet deep, quite a bit above a typical Class VI well at ~ one mile of depth. Besides the sheer depth, injecting this far below sensitive aquifers also allows for multiple geological seals above the injection reservoir to act as fail safes in the event of a leak.
There are specific cases where an injection zone is approved above a USDW by waiver. An example is shown in the right panel of the image below, where a seal, or confining zone, beneath the USDW protects it from the overlying injection zone.
The second reason why we store CO2 at great depth is because it is more efficient. Because they are saturated with saline water, deep reservoirs exert enormous hydrostatic pressure and keep the CO2 in a dense phase, allowing for more efficient storage in the reservoir. CO2 in this phase is referred to as a supercritical fluid. Pressure conditions for this dense phase begin to occur around depths of 0.4 miles. Because most injections occur at depths of one mile or deeper, we can guarantee that the CO2 remains in this more dense state.
What is a supercritical fluid?
Matter is generally found in three states: solid, liquid and gas. But in extreme conditions, rarer states of matter can occur. At temperatures above 88ºF (31.1°C) and pressures in excess of 1,071 psi (72.9 atm), CO2 transitions to a rare state of matter called a supercritical fluid, which exhibits properties of liquids and gases.2USDOE. (n.d.). How can CO2 be stored underground? Retrieved 10/27/2020, from https://www.netl.doe.gov/coal/carbon-storage/faqs/carbon-storage-faqs Supercritical fluids are unique because they have the molecular density of a liquid, but expand to fill their container like a gas. This is ideal when storing CO2 underground as gaseous substances are far easier to inject into reservoirs. However, the main benefit of storing CO2 in a supercritical state is its density.
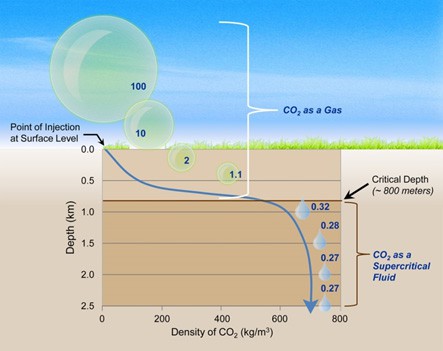
Supercritical CO2 is greater than 300 times more dense than in its gas state! That means that reservoir capacity is increased astronomically when CO2 is stored in its supercritical state. As part of an injection program, CO2 is compressed into a supercritical state before being injected down a well. Conveniently, the reservoirs used to store CO2 are at a depth where these temperature and pressure conditions occur naturally, so no extra effort is required to maintain the supercritical state post-injection.
What could go wrong if CO2 leaks into my water?
The presence of CO2 in water has no ill effects—in fact, if you’ve ever had Perrier or any other carbonated water beverage, you’ve consumed water infused with carbon dioxide! The main concern of CO2 seepage into water sources is the effect that CO2 can have on other chemicals in the aquifer. When CO2 and water mix, they form a small amount of H2CO3, known as carbonic acid. If enough carbonic acid is produced to significantly affect the pH of the water table, it can dissolve minerals in the rock formation and release metals such as arsenic, lead, and mercury. These metals occur in small amounts in our daily diet, but extreme concentrations can damage the nervous system.
Lab tests show that the CO2 contamination has to reach a very high level to release these metals in excess of EPA standards. The presence of multiple low-permeability formations between the CO2 injection zone and shallow freshwater aquifers makes the long-distance migration of fluids between reservoirs very unlikely.3Kelemen, P., Benson, S. M., Pilorgé, H., Psarras, P., & Wilcox, J. (2019). An overview of the status and challenges of CO2 storage in minerals and geological formations. Frontiers in Climate, 1, 9. Additionally, some naturally occurring carbonated water sources (such as Perrier) produce extremely high-quality water. So, even in the case of seepage, the specific geology of the aquifer is the main factor in the risk of damaging water quality.
Images: “Blue Water” by Africa Studio/Shutterstock.com